Unraveling the Microcosm: What REALLY Makes a Molecule a Molecule?
Ever wondered what the world is made of? No, not just the "earth, wind, and fire" kind of stuff, but the *really* small stuff. We're talking about the building blocks of, well, everything! That's where the amazing world of molecules comes in. These tiny particles are more than meets the eye, and understanding the very best definition of a molecule can unlock a whole universe of knowledge.
It's easy to take molecules for granted. After all, we can't see them! But think about it: the water you drink, the air you breathe, even the screen you're reading this on – they're all made up of these teeny, tiny particles, linked together in fascinating ways. Getting a grip on the true definition of a molecule allows us to understand how matter behaves, reacts, and forms the incredible diversity of our world.
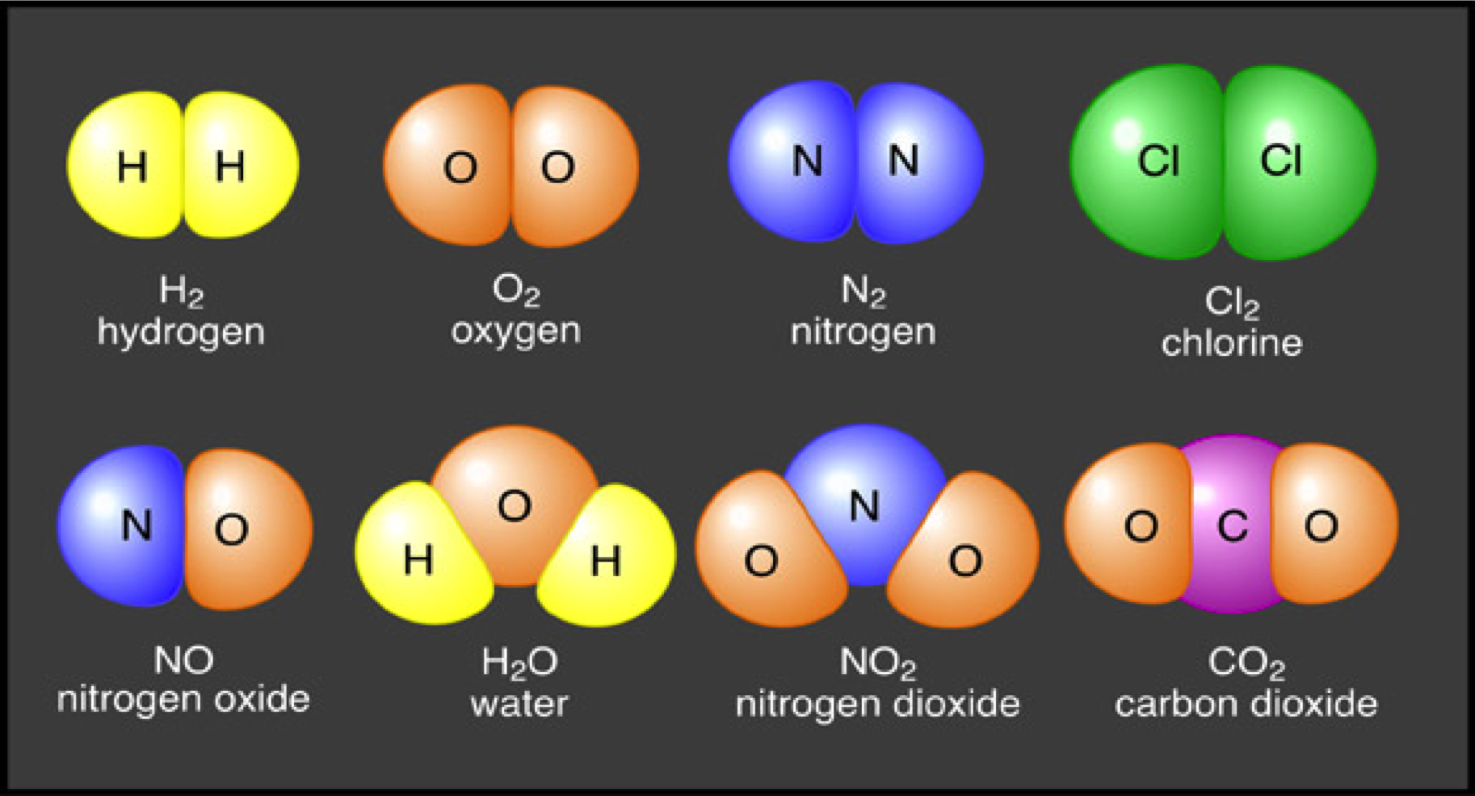
So, how do we define a molecule? In the simplest terms, it's a group of two or more atoms held together by chemical bonds. Think of atoms like individual Lego bricks, each with its own unique properties. When these atoms connect – snap! – they form a molecule, a new structure with its own distinct characteristics.
The journey to understanding molecules goes way back. Ancient Greek philosophers had their theories about atoms, but it wasn't until the 18th and 19th centuries that scientists like John Dalton and Amedeo Avogadro laid the groundwork for modern molecular theory. They realized that substances combine in fixed ratios, hinting at the existence of these fundamental units. With advancements in technology, like powerful microscopes, we've been able to "see" molecules and confirm their existence.
But why does the best definition of a molecule matter so much? Well, it forms the bedrock of chemistry and many other scientific fields. It helps us explain everything from why water boils at a certain temperature to how medicines interact with our bodies. It allows us to engineer new materials with amazing properties, like super-strong plastics and life-saving drugs. The possibilities are endless!
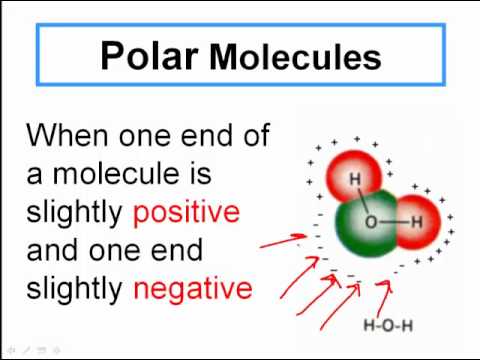
Let's dive into a few examples to make this crystal clear. Take water, probably the most famous molecule out there. It's formed when two hydrogen atoms bond with one oxygen atom (H2O). This simple arrangement gives water its unique properties, like its ability to dissolve many substances and its role as a universal solvent in living organisms.
Or consider something more complex like DNA, the blueprint of life itself. DNA is a gigantic molecule composed of countless atoms arranged in a double helix structure. This intricate design allows it to store and transmit genetic information from one generation to the next.
These examples show how the best definition of a molecule, far from being a dry, academic concept, unlocks the secrets of the world around us and even within us! Understanding molecules is key to advancements in medicine, agriculture, materials science, and countless other fields that shape our future.
So, the next time you see a glass of water, take a deep breath, or marvel at the complexity of nature, remember the molecules! These tiny building blocks are the unsung heroes of our universe, and understanding them opens the door to a world of wonder and possibility.
Unlocking the secrets of fast animals
Decoding the nfl draft landscape a look at player evaluations
The enduring charm of kim seokjins songs




/h20-58e655f93df78c5162ea0a1f.jpg)






-24767-p.jpg?v=0C05EB52-D0AB-4A16-9645-409295BCCD94)
